Background: Since most therapeutic agents for hematologic malignancies are developed and first approved in the US, it is important for each country to review and approve these agents without delay. Recently, the number of drugs developed by non-global companies including biotech start-ups has increased. Most of these non-global companies are located in the US, and typically do not have offices outside of the US. Thus, it is possible that drugs developed by non-global companies are likely to be approved later in countries other than the US, including the EU. However, their characteristics have not been fully evaluated.
Study Design and Methods: Using the public database of the Food and Drug Administration (FDA) from January 2011 to December 2020, new molecular entity (NME) drugs for hematological malignancies that were approved in the US during the relevant period were identified. Cellular products, including chimeric antigen receptor (CAR)-T cells, were excluded from this analysis. NMEs for hematological malignancies were divided into two categories according to the applicant company: NMEs from non-global companies (non-Global group) and NMEs from global companies (Global group). Global companies were defined as those with branches or headquarters in all following regions: the US, the EU, and Japan. The others were classified as non-global companies. Based on the public databases of Japanese regulatory agency (PMDA) and European regulatory agency (EMA), we estimated the rate at which these drugs remained unapproved (unapproved rate) using the Kaplan-Meier method. The unapproved rate of NMEs was compared between the non-Global and Global groups.
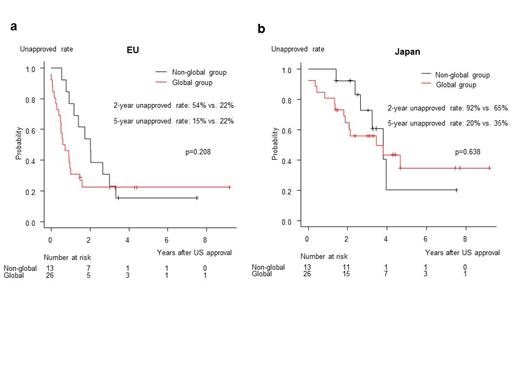
Results: A total of 39 NMEs for hematologic malignancies approved in the US during the study period were identified. Of these, 13 (33%) NMEs were from non-global companies (non-Global group) and 26 (67%) NMEs were from global companies (Global group). The proportion of orphan drug (13/13 vs. 26/26, p=1.000) and priority review designation (12/13 [92%] vs. 22/26 [85%], p=0.648) were not significantly different between the non-Global and Global groups. In the non-Global group, 12 (92%) NMEs were from the companies headquartered in the US, while 10 (39%) NMEs were from the US companies in the Global group. The proportion of pivotal phase Ⅰ/Ⅱ trial used for marketing authorization in the US was significantly higher in the non-Global group (11/13 [85%] vs. 13/26 [50%], p=0.045). The proportion of pivotal trials enrolling Japanese participants was lower in the non-Global group (0/13) than in the Global group (6/26 [23%]), while the proportion was similar in the EU (9/13 [69%] in the non-Global group and 16/26 [62%] in the Global group). In the EU, the unapproved rate 2 years after the US approval was higher in the non-Global group (54% vs. 22%), but the 5-year unapproved rate was rather lower than that in the Global group (15% vs. 22%) (Figure 1a). Similarly, in Japan, the unapproved rate of NMEs in the non-Global group was higher at 2 years (92% vs. 65%), but rather slightly lower than that in the Global group at 5 years (20% vs. 35%) (Figure 1b). After the US approval, 9 (69%) of the 13 NMEs filed by non-global companies had subsequently partnered with or been acquired by the global companies.
Discussion and Conclusion: In both the EU and Japan, drugs in the non-Global group had a higher unapproved rate at 2 years. Notably, 54% of the drugs in the non-Global group were not approved in the EU, even though the participation rate in the pivotal trial used for US approval was similar between the non-Global and Global groups in the EU. However, at 5 years, the unapproved rate had decreased to approximately 20% in both the EU and Japan, presumably due to the fact that lots of the non-global companies were subsequently partnered with or acquired by the global companies.
Disclosures
Matsuda:Kyowa Kirin: Honoraria; Sanofi: Honoraria; Ono Pharmaceutical: Honoraria; SymBio: Honoraria; AbbVie: Honoraria. Nagai:Otsuka Pharmaceutical: Honoraria; Mitsubishi Tanabe Pharma: Honoraria; Takara Bio Inc: Honoraria; Konica Minolta REALM: Honoraria; Nipro: Research Funding. Sugimoto:Chugai Pharmaceutical: Honoraria; Ono Pharmaceutical: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal